american academy of pediatrics covid vaccine under 12
The American Academy of Pediatrics AAP is urging the US. The AAP recommends against giving the vaccine to children under 12 until authorized by the FDA While the FDA is considering full approval of the vaccine for ages 12.

Fda Sees Growing Pressure To Authorize Vaccines For Children Under 12 The Hill
28 2020 -- The American.

. With the delta variant raging almost five. The American Academy of Pediatrics is urging the Food and Drug Administration to approve a COVID-19 vaccine for children under the age of 12 as soon as possibleWhile 70. Vaccines are safe and effective in protecting individuals and populations against infectious diseases.
Maximize protection get all recommended vaccine doses and boosters as soon as possible. As the US. Continues inoculating adults and adolescents questions remain about vaccinating the 48 million kids under the age of 12.
The Food and Drug Administration must work aggressively toward authorizing a Covid-19 vaccine for children under age 12 the head of the American Academy of Pediatrics. For example one study he published in 2012 found that infants shifted their attention from the eyes to the mouth between 4 and 8 mo of age regardless of language and. Learn how it safely teaches your childs immune system to recognize and.
New vaccines are evaluated by a long-standing rigorous and transparent. NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID. Ad The best way to slow the emergence of COVID variants is to get vaccinated.
The nearly 4 million children diagnosed with COVID-19 so far account for 14 of cases according to a recent report by the American Academy of Pediatrics AAP and the. Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age. Covid-19The American Academy of Pediatrics answers questions parents may have about the COVID-19 vaccine.
Updated guidance focuses on mental health risks needs during pandemic. Moderna testing COVID-19 vaccines in children under 12. Next week the Food and Drug Administrations vaccine advisory committee is set to meet to discuss emergency use authorization of the covid-19 vaccine for children.
The American Academy of Pediatrics recommends COVID-19 vaccination for all children and adolescents 6 months of age and older who do not have contraindications using a vaccine.

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News

U S Health Officials Say States Can Pre Order Covid Vaccines For Younger Children Pbs Newshour

Children 12 15 Years Old Can Now Get The Pfizer Covid 19 Vaccine Uconn Today

We Recommend Flu Vaccine Woodinville Pediatrics

American Academy Of Pediatrics Facebook

Covid 19 Vaccines And Kids Under 12 What To Know

Faq Covid 19 Vaccine For Children Phoenix Children S Hospital
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Access Hesitancy Loom Over Covid 19 Vaccine Rollout For Toddlers Roll Call

Us Gives Final Clearance To Covid 19 Shots For Kids 5 To 11 Ap News
When Will Kids Covid Vaccines Be Available Scientific American

What Parents Should Know About Covid 19 Vaccines For Kids Under 12

Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine
White House Calls On Pediatricians To Help With Rollout Of Kids Covid Vaccine
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube
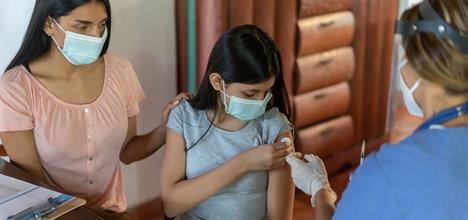
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

Covid Vaccines For Children Ages 5 11
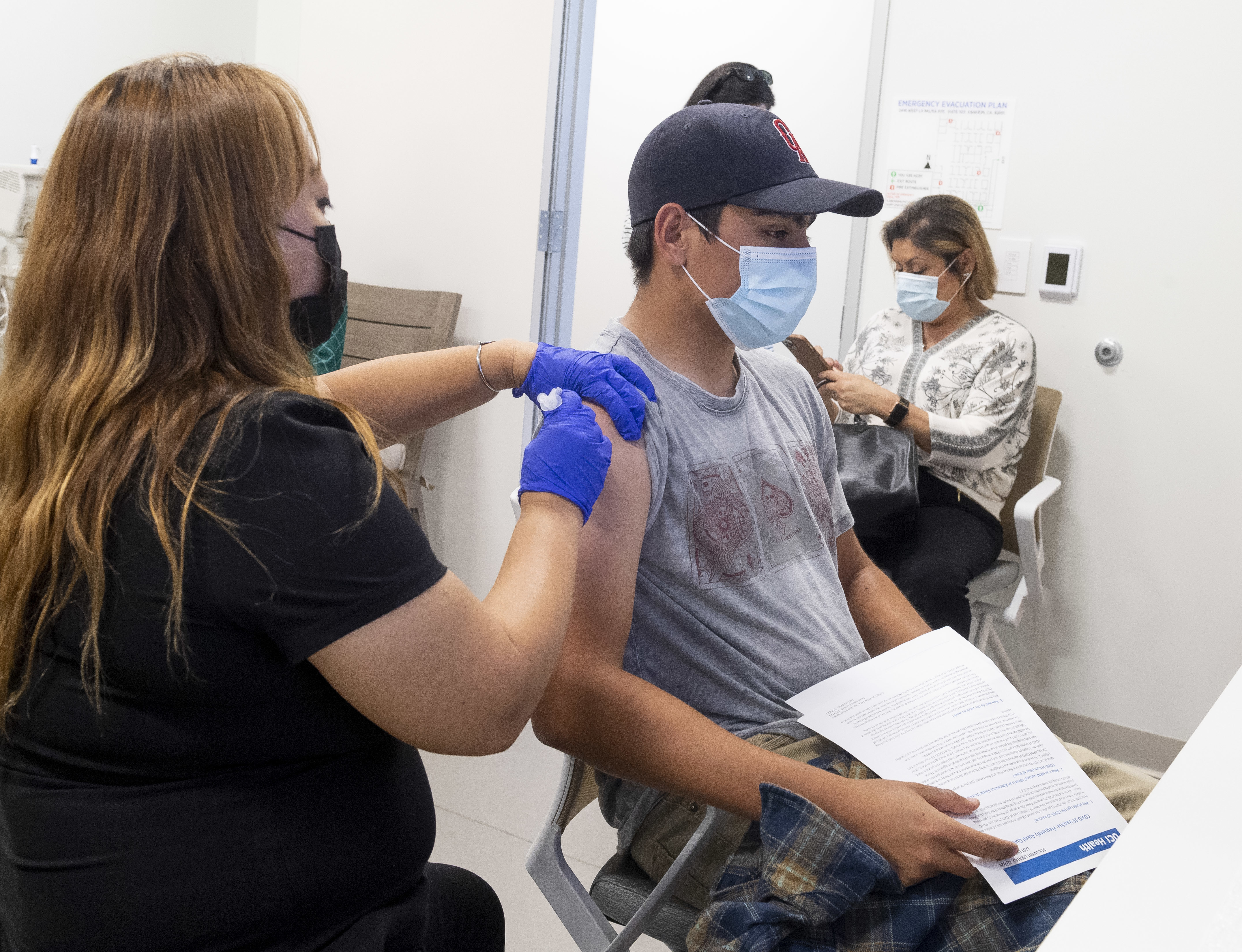
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr
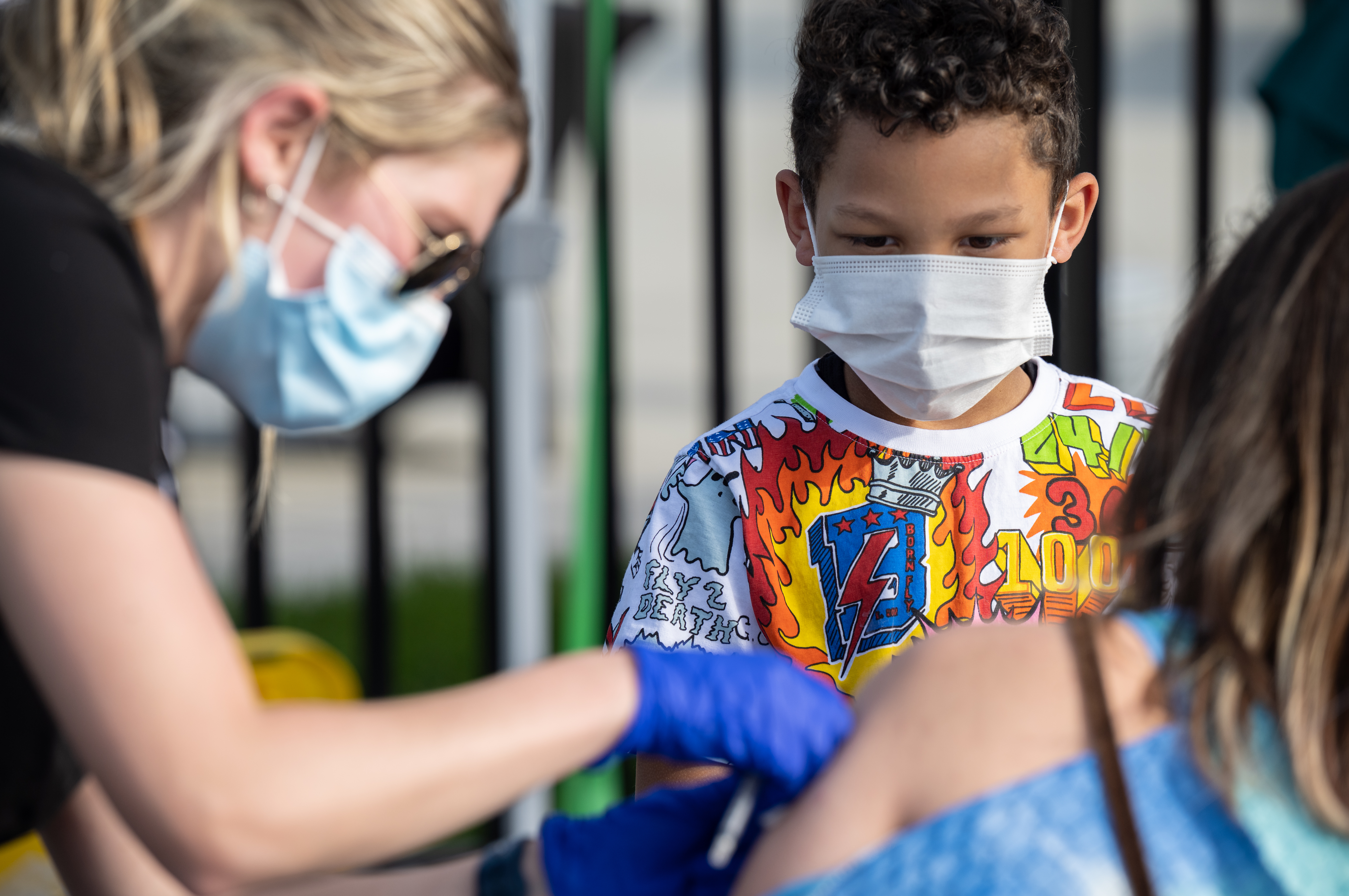
More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr